Archaea metabolism and evolution
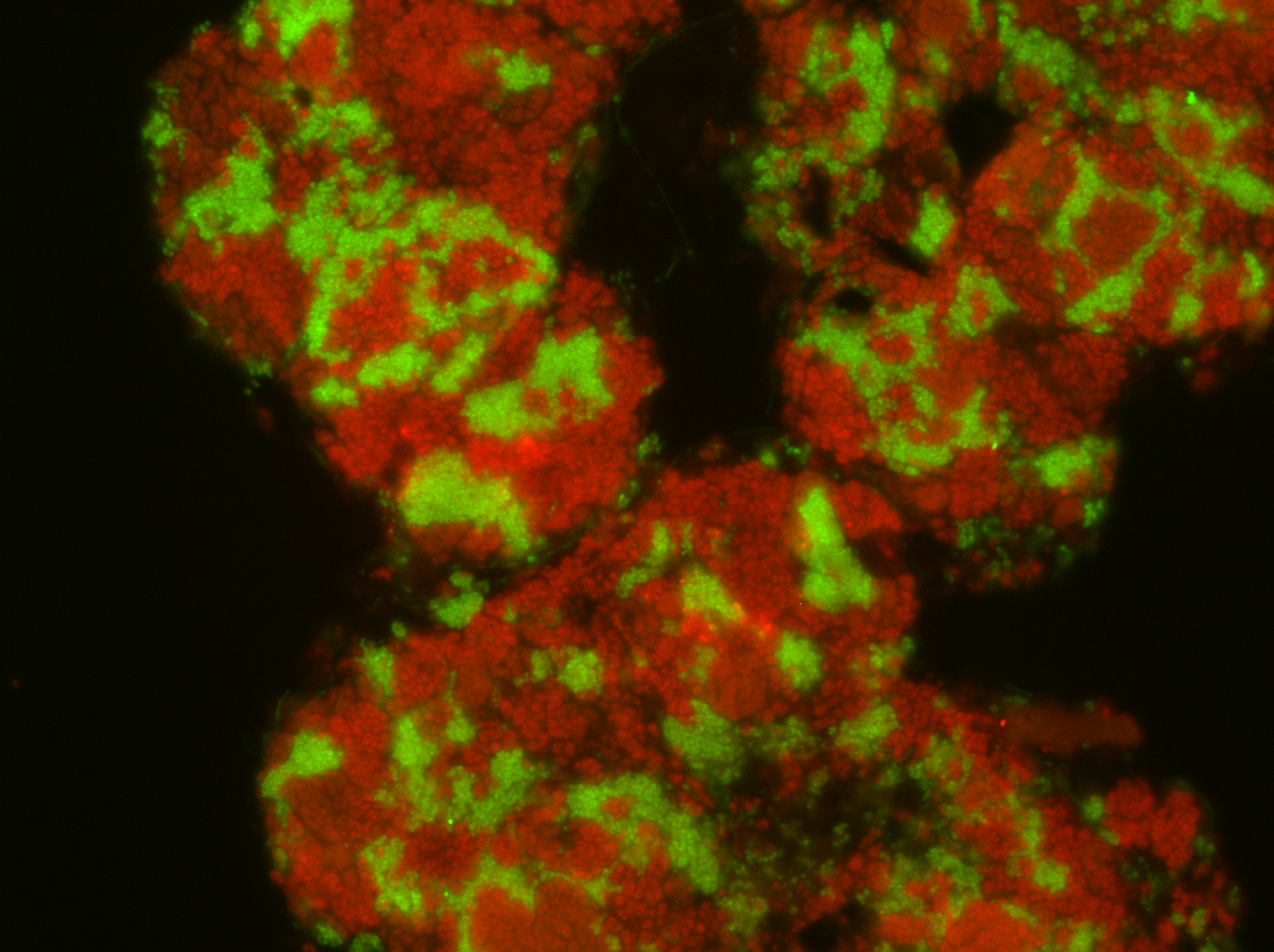
Archaea are one of the three domains of life, but for long their metabolism and evolution have been understudied in comparison to bacteria. Archaea play an important role in global biogeochemical cycles. Especially relevant is their role in the carbon cycle because of their unique capability to produce methane as metabolic end product, a potent greenhouse gas. The key enzyme of methanogenesis is the methyl coenzyme M reductase (MCR) which catalyzes the last step of the methanogenesis pathway: the reduction of a methyl group to methane. Some archaea called anaerobic methane-oxidizing archaea (ANME) can reverse the methanogenesis pathway and oxidize methane. Their MCR catalyze the reaction in the opposite direction and activate methane to methyl groups bound to a coenzyme M. My research career has focused on the field of environmental microbiology, especially in the role of archaea in the anaerobic degradation of hydrocarbons, where I have made ground-breaking contributions in relation to
- Cultivation of novel hydrocarbon-degrading archaea
- Genomic characterization of methanotrophic ANME archaea.
Novel hydrocarbon-degrading archaea

During my PhD, I discovered that divergent MCRs present in archaea activate non-methane hydrocarbons under anoxic conditions.This was an extremely striking finding, since MCR was thought to exclusively catalyze methane metabolism. The combination of classic microbiology with modern multi-omics approaches enabled me to prove my genomic-derived hypothesis with physiological experiments and develop a conclusive model on the functioning of this organism.
This model has crystallized in a novel pathway to degrade non-methane hydrocarbons using the alkyl-CoM activation mechanism catalyzed by divergent MCRs, now renamed as alkyl coenzyme M reductases (ACRs). This finding has paved the way for a wider understanding of the environmental role of archaea in subsurface environments and the function of MCR/ACR. In this regard, I have been involved in many projects that have shown that ACRs appear in different archaeal groups and can activate different kind of hydrocarbons from ethane to long-chain alkanes and alkyl-aromatics.
You can check the video below to understand better how these archaea live!
Relevant publications:
- Thermophilic archaea activate butane via alkyl-coenzyme M formation. Nature (2016)
- Non-syntrophic methanogenic hydrocarbon degradation by an archaeal species. Nature (2022)
- Establishing anaerobic hydrocarbon-degrading enrichment cultures of microorganisms under strictly anoxic conditions. Nature Protocols (2018)
- Anaerobic Degradation of Non-Methane Alkanes by "Candidatus Methanoliparia" in Hydrocarbon Seeps of the Gulf of Mexico. mBio (2019)
- Anaerobic Degradation of Alkanes by Marine Archaea. Annual Review of Microbiology (2022)
- Candidatus Alkanophaga archaea from Guaymas Basin hydrothermal vent sediment oxidize petroleum alkanes. Nature Microbiology (2023)
- "Candidatus Ethanoperedens," a Thermophilic Genus of Archaea Mediating the Anaerobic Oxidation of Ethane. mBio (2020)
Methanotrophic ANME archaea

I have investigated the metabolism of ANME archaea, which are responsible for the anaerobic oxidation of methane. ANME represent a functional and polyphyletic clade formed by three different phylogenetic groups ANME-1, ANME-2 and ANME-3. The three of them are closely related to methanogenic organisms and use a reversal of the methanogenesis pathway to oxidize methane. The fact that both methanogens and ANME share their core pathway has raised questions about the evolutionary emergence of ANME and about the mechanisms that drive the functioning of the pathway in the reductive or the oxidative direction. By using metagenomics, I described a deep-branching, thermophilic family of the ANME-1 group, '´Candidatus Methanospirareceae', which is closely related to the sister archaeal groups of alkane oxidizers. Global phylogeny analysis revealed that hydrogen metabolism is present in ANME-1 as an ancient trait, but it has been differentially lost during lineage diversification. Our omics' analysis allowed to retrieve the expansive ANME-1 virome revealing that some ANME-1 specific genes (i.e. ThyX) were acquired by virus-mediated gene displacement.
Besides, I participated in the first genomic atlas of the diverse ANME groups, where we found unique genomic features that separate ANME organisms from their methanogenic counterparts like bioenergetic complexes and large multiheme cytochromes. Interestingly, these features are not the same between ANME clades, confirming a separate origin of the different ANME clades during evolution. More recently, I was involved in a publication of ANME-3, the least known of the ANME groups. We were able to identify different steps that allowed the transition from methanogenesis to methanotrophy in this clade. Finally, I was recently involved in characterizing the MCR enzymes of two ANME-2 groups.
Relevant publications:
- Atomic resolution structures of the methane-activating enzyme in anaerobic methanotrophy reveal extensive post-translational modifications. Nature Communications (2025)
- Identification of key steps in the evolution of anaerobic methanotrophy in Candidatus Methanovorans (ANME-3) archaea. Sciences Advances (2025)
- Evolutionary diversification of methanotrophic ANME-1 archaea and their expansive virome. Nature Microbiology (2023)
- Comparative genomics reveals electron transfer and syntrophic mechanisms differentiating methanotrophic and methanogenic archaea. PLoS Biology (2022)
- Anaerobic Degradation of Alkanes by Marine Archaea. Annual Review of Microbiology (2022)
- Physiological
potential and evolutionary trajectories of syntrophic sulfate-reducing
bacterial partners of anaerobic methanotrophic archaea. PLOS Biology (2024)